Description
The demands on laboratory management are constantly increasing: while order and sample volumes are rising on the one hand, the requirements of accreditation, management, customers, employees and documentation are growing on the other. The goal of the laboratory database is to master this balancing act: as a central system, it is the place where all information relevant to the laboratory is systematically structured and clearly linked in a central database and can be accessed at any time and from anywhere.
The laboratory is at the center of all developments. With a clearly regulated update policy, new requirements are therefore also available as standard for all customers. Support at eye level is guaranteed in daily contact: All employees of the laboratory database have many years of laboratory experience.
The laboratory database has been developed from the ground up to meet accreditation requirements and is currently in use by over 3000 users in contract laboratories, operating laboratories, medical diagnostic laboratories, commercial laboratories, research laboratories, food laboratories, QA laboratories, veterinary laboratories, and water and environmental laboratories.
Analytics and order processing are closely linked to the modules for test equipment management, document management, employee training, assessments, qualifications, material and batch management, and risk and opportunity analyses to meet the documentation requirements of DIN EN ISO 17025.
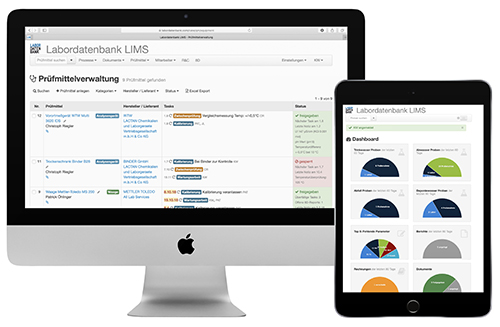
We recommend that interested parties have an initial discussion with one of our LIMS experts and then test their own demo version extensively and independently. Please contact us for this under the data given here.
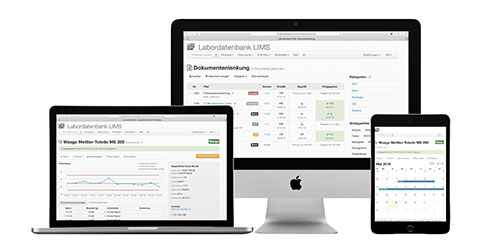
You can find more information here.




