Description
With its modular structure, the Compact.Net software consistently supports you in all analyses, tests and examinations in the laboratory and thus enables holistic sample management in a modern LIMS. Whether the acquisition and management of samples in the food sector, the planning, development and selection of procedures and experiments in pharmaceuticals or the continuous analysis of bath parameters in electroplating companies: Compact.Net enables you to manage the sheer volume of information and strict data security requirements of a LIMS across industries. From handling deviating analysis results such as Out-of-Specification (OOS) Results, Out-of-Expectation (OOE) Results or Out-of-Trend (OOT) Results, to managing Certificates of Analysis (CoA), Compact.Net gives you the best possible support.
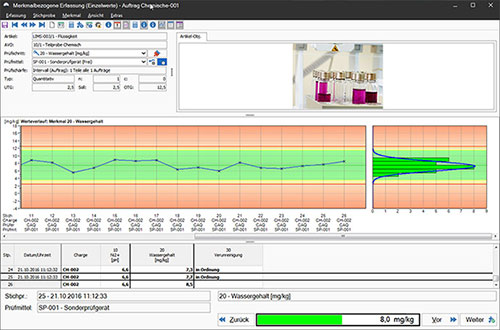
Analytics
From sample receipt, sample preparation and distribution, through planning and execution of analysis, to statistical analysis and creation of control charts, Compact.Net offers all the tools needed for data acquisition, preparation and evaluation. In order to comply with information security and a wide range of traceability requirements, the software also features tools for traceability with comprehensive batch tracking as well as audit-proof management of analysis specifications. Thanks to FDA-compliant electronic signatures, change control and consistent data monitoring in terms of data integrity, Compact.Net enables safe working even in validated environments.
Information technology integration of the laboratory into the company
Thanks to the bidirectional connection of laboratory equipment and IT systems such as ERP systems, the laboratory no longer operates in isolation. This is because the exchange of digital information provides a direct overview not only of the entire situation in the laboratory, but of the entire company. Experience has shown that the efficiency gains achieved by our customers in their laboratory workflows amount to at least around 30 percent, and the paper savings average 20-50,000 pages per year. Compact.Net is already enabling the paperless laboratory of tomorrow.
Modular design for easy entry into electronic laboratory management
In terms of future viability, the software solution is convincing in that the system can be successively expanded with additional modules after the introduction of the Compact.Net solution for laboratory analyses. In addition to the digital implementation of LIMS, records management, and audit trail, the CAQ.Net software also offers modular solutions for:
- Process and workflow management
- Document Management
- Training management / e-learning
- Task and escalation management
- Supplier Management
- Audit Management
- Change Management
- Key figure management
- Test planning / quality inspection / SPC / LIMS
- Initial sample inspection
- Gauge management
- Preventive maintenance
- Risk management / FMEA
- Complaint management / CAPA
Thus, the solution enables much more than a conventional LIMS and is therefore especially interesting for manufacturing companies.
You can find more information here.




